Training: How to Use CADScor®System
How to Perform a CADScor®System Assessment

Preparation
- The patient should relax for at least five minutes before the test.
- Identify the IC4-L region on the patient’s chest and remove hair if needed.
- Attach CADScor®Patch to the sensor using the assembly tool.
- Add the patient’s clinical risk factors in CADScor®System
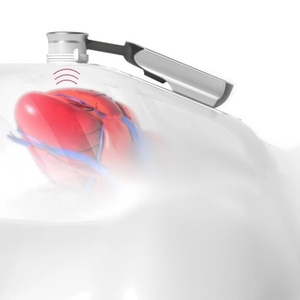
Application and Recording
- Place patch with sensor at IC4-L, 2 mm off the sternum.
- Instruct the patient on the recording sequence and how to breathe.
- Start recording. A Pre-recording is conducted, followed by a main recording lasting less than three mins.
- Guide patients on when to hold their breath.
- Be quiet during recording.

Data Analysis
- The recorded sounds are automatically filtered and processed in the device.
- The CAD-score result is displayed on the screen as a number between 0 and 99.
- The same patch can be used up to four times if not removed from the patient’s chest.
- The system will produce a QR code with the patient’s score.
- This can be shared with the patient and added to their records. *
The CADScor®System: How it Works
CADScor®System is a point-of-care diagnostic aid that uses highly sensitive acoustics and advanced computational processing to analyze the patient’s coronary blood flow. The system calculates a patient-specific CAD-score, and couples it with risk factors to rapidly indicate the patient’s risk of significant coronary stenosis for immediate risk stratification, prior to potential secondary evaluation.1 CADScor®System has been used in over 40,000 patient assessments 2 and has CE-marking and FDA De Novo clearance.
Instructional Videos
CADScor®System: Intended Use
CADScor®System: First time setup
CADScor®System: Preparation for use
CADScor®System: The Test Recording

CADScor®System Research, Development and Clinical Studies
CADScor®System is approved in Europe with CE-marking and has received FDA De Novo clearance as a diagnostic aid for symptomatic patients with suspected CAD. Multiple clinical studies have been published to demonstrate the CADScor®System performance.

- User manual US-FDA v.12.Y, prevalence 10,7%
- Based on commercial patch use since 2017
*During patient consultation, physicians should use the CADScor®System as a diagnostic aid along with an evaluation of patient symptoms and past medical history.